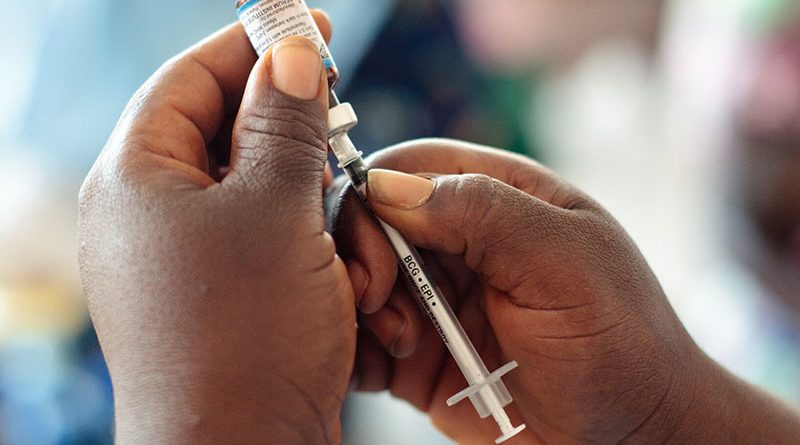
KENYA’S oxygen production firm Hewatele is doubling production this year to keep up with surging demand from hospitals that are treating critically ill COVID-19 patients, the company said.
Demand for the commodity has more than doubled to 880 tonnes from 410 tonnes before the pandemic, the ministry of health said, causing a steep shortage due to lack of installed capacity.
The East African nation is confronting a severe fourth wave of COVID-19 infections that is putting pressure on health facilities.
“This country doesn’t have capacity to put 2,000 patients under high flow oxygen at the same time. We need to do something urgently,” said Bernard Olayo, founder of the company.
Hewatele plans to invest $3.5 million to double production to two tonnes a day by year-end, Olayo said.
The firm produces oxygen by the chemical method, using a naturally occurring salt to separate nitrogen from the air.
“It (chemical method) has limitations because you can’t produce a lot of oxygen,” Olayo said.
To resolve that issue, Hewatele will invest another $15 million next year to build an air separation unit to produce liquid oxygen, which will lead to a ten-fold output increase to 20 tonnes per day, he said.
Kenya has only one such plant, which belongs to industrial gases firm BOC, said Olayo, who was inspired to produce oxygen for hospitals by his experience as a young government doctor in the west of the country two decades ago.
“Children would die of pneumonia, not because we didn’t know what to do, but because we didn’t have oxygen,” he said.
Hewatele’s expansion is being financed by a combination of debt and equity from international financiers, he said.
But the process will not be easy since the pandemic has driven up demand for oxygen and for components used by oxygen plants.
“Getting oxygen compressors is very difficult,” Olayo said.
Hewatele, which supplies 200 hospitals with oxygen, has three production plants around the country to help keep costs down. Still, a litre of liquid oxygen costs 120-150 Kenyan shillings, compared with 10 shillings internationally.
“The demand far outstrips the supply,” said Olayo.
A leading Olympic Games health adviser said on Saturday that Tokyo 2020 had shown the COVID-19 pandemic could be beaten and would provide data to help countries around the world battle the coronavirus.
The Olympics in Tokyo had shown that measures such as social distancing, mask-wearing, hand sanitising along with testing and tracing worked when implemented as a package, Brian McCloskey, the chair of the Game’s Independent Expert Panel, said at a press briefing.
“We have shown it is possible to keep a pandemic at bay and that is a very important lesson from Tokyo to the rest of the world,” he said.
The Tokyo 2020 organiser earlier said that it had recorded 404 Games-related COVID-19 cases since July 1.
Health data collected during the two weeks of the Games, including inside the athletes village, would be analysed and published so countries could use it to help plan their responses to the coronavirus, McCloskey said.
NAIROBI, July 22 (Reuters) - Global pharmaceutical firms should license production of COVID-19 vaccines in Africa rather than just do piecemeal contract deals, an African Union special envoy said on Thursday.
AU coronavirus envoy Strive Masiyiwa was speaking a day after Pfizer and BioNTech announced a "fill and finish" deal with South Africa's Biovac Institute under which it will carry out the final stages of vaccine manufacturing where the product is processed and put into vials. read more
Pfizer and BioNTech will handle drug substance production at their facilities in Europe. Medecins Sans Frontieres (MSF) has called the arrangement "restrictive" and said much more is needed to support vaccine independence in Africa.
"We want to make clear to all suppliers ... if you want a long-term future with us now, you produce from Africa," Masiyiwa said.
Africa, which is battling a third wave of infections, has administered just 60 million vaccine doses in a population of 1.3 billion due to restrictions on shipments from vaccine producing nations like India.
Many African nations rely on global vaccine sharing scheme COVAX or donations from countries like China and India.
"For regions left behind in the vaccine race to be self- sufficient, they need access to all of the components of vaccine production," said Lara Dovifat, manager at MSF's access campaign, which is seeking equitable vaccine access.
Matshidiso Moeti, the head of the World Health Organization in Africa, called for local production of vaccines so Africa can tackle future outbreaks.
"We are looking beyond this crisis," she told a news conference.
Johnson & Johnson (JNJ.N), whose vaccine is administered through a single shot, also has a "fill and finish" deal with South Africa's Aspen Pharmacare (APNJ.J).
J&J is on track to supply members of the African Union with 400 million vaccine doses by September next year, Masiyiwa said.
Around 6 million doses will be delivered to 27 nations that have paid their share through the end of August, Masiyiwa said, with another 18 finalising loans from the World Bank and other global lenders before they make payment.
Deliveries will rise to an average of 10 million a month from September, increasing to 20 million in January until the order is fulfilled by September next year.
The balance of Africa's vaccine requirements will come from donors including COVAX, Masiyiwa said, adding that local production is the real answer.
"If you want land we will give you. If you want to own everything 100%, we don't mind, just produce from the African continent," he told the firms.
Our Standards: The Thomson Reuters Trust Principles.
Companies in Brazil and Senegal will produce antigen rapid tests for diagnosing Covid-19 from early 2022 under tech transfer agreements aimed at boosting availability in Latin America and Africa, international agencies said on Thursday.
High-quality antigen tests are the primary diagnostic tool for detecting active infection in poorer settings where molecular testing is not available, the global alliance for diagnostics FIND and health agency UNITAID said in a statement.
Agreements have been signed to transfer know-how from U.S.-based diagnostics company DCN Dx to WAMA Diagnóstica in Brazil, and from Bionote and Britain's Mologic -- known for the development of the clear blue pregnancy test -- to DIATROPIX of the Institut Pasteur de Dakar, the statement said.
It announced a separate partnership for commercialisation and distribution between Xixia Pharmaceuticals, a South African subsidiary of the generic drugmaker Viatris (VTRS.O), and Guangzhou Wondfo Biotech Co Ltd (Wondfo).
"Expanded production capacity in local and regional hubs is critical to ensuring that healthcare providers in low- and middle-income countries can implement effective testing strategies to contain the spread of the virus,” said UNITAID spokesman Herve Verhoosel.
WAMA Diagnostica is expected to generate test volumes of 2 million per month, with a ceiling price of $2.00 per test, he said.
The partnership between Wondfo, based in Guangzhou, China, and Xixia Pharmaceuticals, has the potential to produce 144 million tests per year at a ceiling price of $2.50 each, he added.
In Senegal, DIATROPIX will seek regulatory approval for the tests transferred from Mologic and Bionote and commercialise them under its own brand, aiming to make 2.5 million tests per month in 2022, the statement said.
Some 144 countries will be eligible for procurement through the ACT-A (Access to Covid-19 Tools Accelerator) scheme, it said.
Source - The East African